BME Professor’s Paper on Controlling Neurons With Light Featured in Nature Electronics
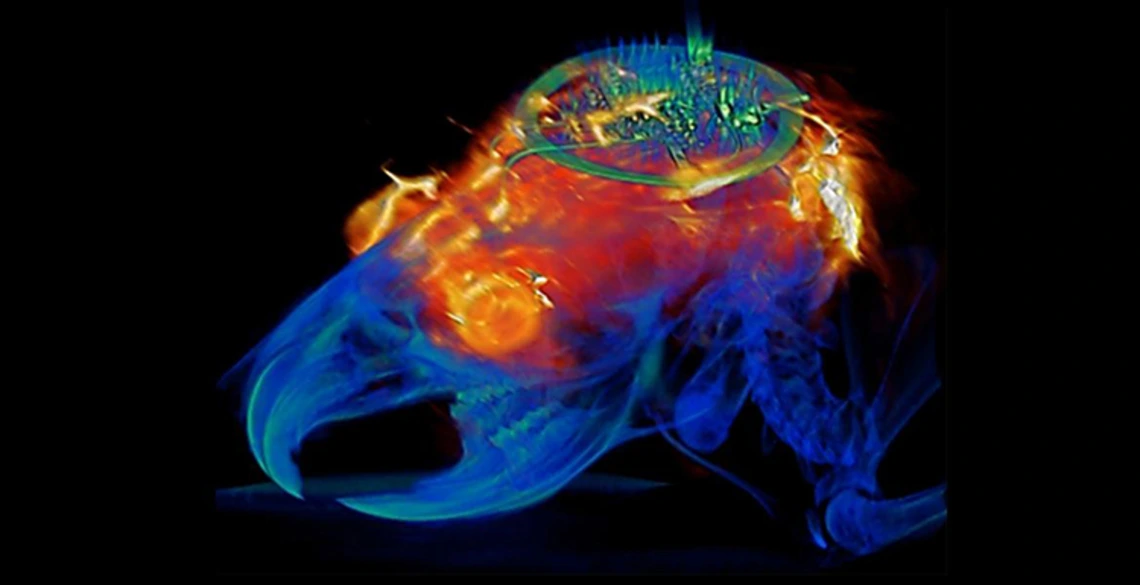
Biomedical engineering professor Philipp Gutruf is first author on the paper "Fully implantable, optoelectronic systems for battery-free, multimodal operation in neuroscience research," published in Nature Electronics.
Optogenetics is a biological technique that uses light to turn specific neuron groups in the brain on or off. For example, researchers might use optogenetic stimulation to restore movement in case of paralysis or, in the future, to turn off the areas of the brain or spine that cause pain, eliminating the need for -- and the increasing dependence on -- opioids and other painkillers.
“We’re making these tools to understand how different parts of the brain work,” said Gutruf, who is also a member of the BIO5 Institute. “The advantage with optogenetics is that you have cell specificity: You can target specific groups of neurons and investigate their function and relation in the context of the whole brain.”
In optogenetics, researchers load specific neurons with proteins called opsins, which convert light to electrical potentials that make up the function of a neuron. When a researcher shines light on an area of the brain, it activates only the opsin-loaded neurons.
The wireless, battery-free implants are powered by external oscillating magnetic fields, and, despite their advanced capabilities, are not significantly larger or heavier than past versions. The research described in the paper also demonstrated that animals implanted with these devices can be safely imaged with computer tomography, or CT, and magnetic resonance imaging, or MRI, which allow for advanced insights into clinically relevant parameters such as the state of bone and tissue and the placement of the device.
